Urease: The Ultimate Therapeutic Target for Helicobacter Pylori- Juniper Publishers
Juniper Publishers- Journal of cell science
Abstract
Gastric ulcer and carcinoma is quite frequent
throughout the globe. The most prevalent causative agent of gastric
ulcer and carcinoma is a gram-negative bacterium, Helicobacter pylori (H. pylori).
The ineffectiveness and side effects of approved drugs as well as
antibiotic resistance is a major problem in the treatment of H. pylori. H. pylori possess uncommon urease enzyme which catalyzes the hydrolysis of urea. Urease is necessary for colonization of H. Pylori
in the gastric mucosa and is a potent immunogen that elicits a vigorous
immune response. As urease acts as both colonization and virulence
factor, new inhibitor of urease from plant sources with no/less adverse
effect is vital to efface H. pylori.
Keywords: Helicobacter pylori; Urease; Virulence; Inhibitor; Antibiotic resistance Introduction
H. pylori infection is common all over the
world. Surviving in stringent conditions of low pH in human stomach is
not feasible for most of the microorganisms. However, unlike other
organisms' gram-negative (H. pylori) can "customize" its
surrounding to make it comfortable for survival. And this survival
tactic of the bacterium leads to chronic gastritis and plays important
role in peptic ulcer disease, gastric carcinoma, and gastric lymphoma [1,2].
Consequences of H. pylori infection
Gastric cancer had been marked as a prominent cause of cancer related death with above 95% relation with infection by H. Pylori [1]. 70 to 90% of the residents of developing countries act as host of H. pylori on the other hand as in advanced countries, the predominance of infection is less [3].
Among infected individuals, approximately 10% develop severe gastric
lesions such as peptic ulcer disease, 1%-3% progresses to Gastric
carcinoma. Gastric carcinoma represents the second most frequent cancer
in the world [4,5].
Gastric carcinogenesis is a multifactorial process and it results from
interaction of the several factors that are related to diet,
environment, genetic susceptibility, and Helicobacter pylori infection. Substantial epidemiological evidence exists for an increased risk of gastric carcinoma with H. pylori infection [6]. This carcinogenesis by H. pylori
consists of two pathways. The direct pathway effects on gastric
epithelial cells, by alteration of DNA and cellular proteins. And the
indirect pathway works by initiating inflammation by innate and adaptive
immunity [1].
Action of the bacterial associated protein cagA, its peptidoglycan,
VacA toxin, sialic acid-binding adhesion (SabA), outer inflammatory
protein (OipA), duodenal ulcer promoting gene (dupA) and the flagella
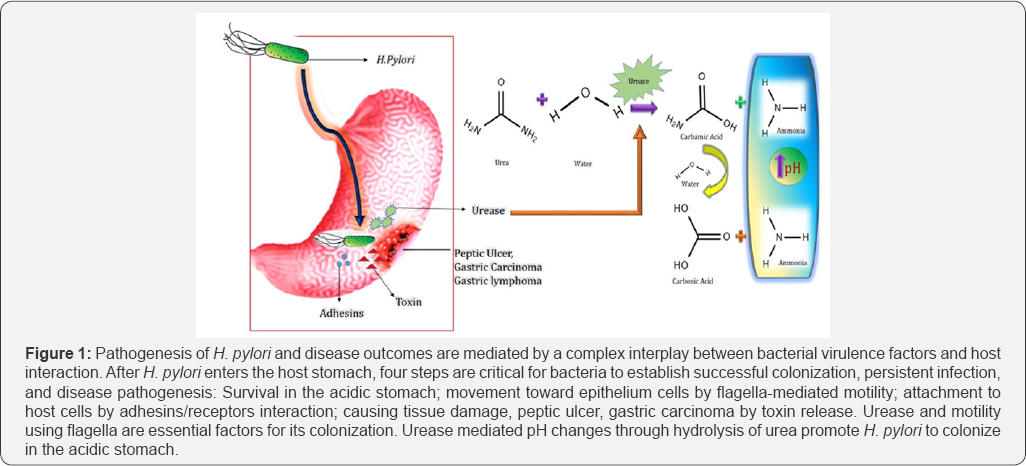
altogether plays eminent roles in development of carcinogenesis [2]. Apart from these, a crucial role of gastritis is played by the unique feature of H. pylori; the urease enzyme [2].
Unique H. Pylori Urease in Action
Urea aminohydrolase also known as urease is a nickel-
containing hexameric molecule. It catalyzes the hydrolysis of urea into
ammonia and carbamic acid (Figure 1).
Simultaneously carbamic acid decomposes into carbonic acid and a second
ammonia molecule. Ammonium molecule being a weak base elevates the pH,
hence making the surrounding for H. pylori comfortable to colonize in harsh acidic condition [7,8].
Significant amount of ammonium also promote cytotoxic chemical such as
monochloramine which causes tissue damage. Moreover, the carbonic acid
which is another product of the catalytic process diminishes the
antimicrobial activity of peroxynitrite, a metabolite of nitric oxide.
Therefore protecting the bacteria in every way possible [9].
All these features of the bacteria become evident due to the presence
of its exclusive urease enzyme. This enzyme is composed of only two
protein subunits UreA and UreB with ratio of 1: 1, unlike other
ureolytic bacteria which consists of another UreC subunit. It is to be
noted that bacterial ureases are activated by supplementary proteins
UreD, UreE, UreF, UreG,and UreH through a complex process [7,8].
Targeting these formulation of drug aimed towards deregulation in
colonization proteins as well as their respective genes can lead to an
ideal of H. pylori (Figure 1).
Current Therapeutics against H. Pylori
H. pylori is usually treated with triple
therapy consisting of clarithromycin, amoxicillin and a proton pump
inhibitor. Due to hike in antimicrobial resistance sequential, quadruple
regimes had been introduced recently. However, newer quadruple
combinations still result in a high frequency of side-effects and some
studies report low cure rates [10].
The antibiotics administered, usually act on the bacterium, on the
other hand inactivation of urease enzyme is not yet totally exploited.
At present Palmatine, Bis (N-methylaminomethyl) phosphinic acid, and
some derivatives of 3-Arylpropionylhydroxamic acid, pyrogallol and
catechol were observed to suppress urease by acting on active site [11-14]. Acetohydroxamic acid (AHA), which is used to treating H. pylori by inhibiting urease enzyme, also exhibits severe side effects [15].
Urease: The Ultimate Target for H. Pylori
It is the enzyme 'urease' and its activity by which H. pylori
colonize in the host. Therefore if the urease can be arrested, the
bacteria would not have the advantage of inhibiting the acidic condition
of the stomach. Though the available drugs work on various factors of
urease as a whole, attention on its proteins UreA and UreB as well as
UreD, UreE, UreF, UreG, and UreH, hence their respective genes could be
focused on. Poor patient compliance with the available eradication
therapy due to the development of side-effects may be associated with
higher treatment failure rates and may favor the development of
antibiotic-resistant strains of H. pylori. Therefore, H. pylori treatment still remains a challenge due to antibiotics resistance, side-effect and cost, mainly in developing countries [16-18].
Thus, developing alternative therapeutics with higher
efficacy and lower side effects is a burning necessity. In this
scenario, phytochemicals could be an effective alternative [1922].
Till date, very few studies have been conducted on plant derived
compounds for the identification of novel therapeutics against H. pylori [11,14].
If urease is widely explored to inhibit/ deactivate/ distort the enzyme
through bioactive compounds from plant source(s), it can lead to a new
hope to "un-root" H. pylori and prevent further infection.
For
more Open access journals please visit our site: Juniper Publishers
For
more articles please click on Journal of Cell Science & Molecular Biology




Comments
Post a Comment