Evaluation of antioxidant and cytotoxic properties of Vernonia amygdalina- Juniper Publishers
Juniper Publishers- Journal of Cell Science
Abstract
The present investigation was carried out to evaluate the antioxidant activity and cytotoxic properties of Vernonia amygdalina.
The free radical scavenging activity using a stable radical; 2,
2-Diphenyl-1-picryl hydrazyl, lipid peroxidation assay (DPPH), and
nitric oxide inhibitory assay gave the highest percentage inhibition as
74.55±1.07%; IC50 = 1.831, 60.42±0.11; IC50 = 3.84 ± 1.03 and 71.26±0.48; IC50
= 0.99mg/ml, respectively. This is comparable to the standards
quercetin used (P>0.05). In addition; total phenol, total flavoniods,
anthocyanin and proanthocyanidine of the extract were determined using
established methods. The results obtained justify the scavenging
activity of the extracts. Furthermore, the extracts possessed very low
cytotoxicity to brine-shrimp lethality test, when compared with the
reference standard (Potassium dichromate, LC50 = 0.003±μg/mL). The results obtained in the study indicate that V. amygdalina can be a safe potential source of natural antioxidant agent; used as a neutralcetical/functional food..
Keywords: 2; 2-Diphenyl-1-picryl hydrazyl; Antioxidant; Cytotoxicity; Veronia amygdalinaisIntroduction
Vernonia amygdalina is a shrub that grows
predominantly in the tropical Africa. Leaves from this plant serve as
food vegetable and culinary herb in soup [1]. Anecdotal evidences
suggest the use of V. amygdalina in the treatment of feverish
condition, cough, constipation, hypertension and related vascular
diseases as well as diabetes. Photochemical screening of this plant
leaves extracts showed the presence of Saponins, riboflavin,
polyphenols, sesquiterpene and flavonoids [2]. Strong antioxidant
activities involving flavonoids extracted from V. amygdalina and its saponins have been reported to elicit anti-tumoral activities in leukemia cells [3]. In addition, peptides from V. amygdalina
are known to be potent inhibitor of mitogen activated protein kinase
(MAPK) which is involved in the regulation and growth of breast tumour
[4].
Previous studies have shown that a good number of
plants have antioxidant activities that could be therapeutically
beneficial. Consequently, antioxidant agents of natural origin have
attracted special interest because of the potential they hold in the
maintenance of health and protection of some age related degeneration
disorders, such as coronary heart disease and cancer, neurodegenerative
disease [5-7].
Although, antioxidants from natural sources are
beneficial, it is pertinent to know their bio-safety. In this regard,
the brine shrimp lethality assay is considered a useful tool for
preliminary assessment of toxicity of plant extracts; a suggested
pharmaco logical screening method in plant extracts. It has been used
for the detection of fungal toxins, plant extract toxicity. The shrimp
lethality assay was proposed by Michael and co-workers in 1956, and
later developed by Vanhaecker and his group in 1981. This is based on
the principle, whereby the kill laboratory-culture of an invertebrate, Artemia salina
L (the brine shrimp larva) following exposure to a varied concentration
of plant extracts, heavy metals, cyan bacteria toxins and pesticides,
is assessed for toxicity [8]. The purpose of this study is to evaluate
the acute toxicity and antioxidant properties of V. amygdalina in relation to its use as a neutralcetical.
Materials and Methods
V. Amygdalina: Fresh leaves of V. amygdalina
were collected from the University Village, Kogi State University,
Nigeria. The plant material was identified and authenticated by
taxonomist in the Department of Botany, Kogi State University, where the
voucher specimen (VA-111) was deposited. Fresh leaves of V. amygdalina were
air dried under room temperature until a constant weight was obtained.
Thereafter, the leaves were milled to a coarse powder with the use of
laboratory Mortar and Pestle. After this, 20g of the plant powder was
weighed into a volumetric flask and then extracted using 200mls of
distilled water for 72 hours. The crude extract was obtained by
concentrating the water soluble extract using rotary evaporator at 45
°C. The working solution of extract was prepared by weighing out 0.02g
of crude extract accurately
and dissolved it in 20ml of distilled water to give an effective
concentration
of 1mg /ml.
Radical scavenging activity
In order to determine the antioxidant properties of the plant,
radical scavenging activities of the leaves extract, was determined
using the stable radical DPPH (2, 2-diphenyl-1 piccrlhydrazyl
hydrate) according to the method of Blois (1958) as describe by
Babalola and co-workers [9]. The principle is based on the reaction
of DPPH, and an antioxidant compound to generate hydrogen,
which is reduced (DPPH + RH → DPPH2 + R). The observed
colour change from deep violet to light yellow was measured at
517nm. To 1ml of varied concentrations (0.5, 0.25, 0.125, 0.0625,
0.003125mg/ml) of the extract or standard, was added 1ml of
0.3mM DPPH in methanol. The mixture was vortexed, and then
incubated in a dark chamber for 30minutes. Thereafter the absorbance
was read at 517nm against a DPPH control containing only
1ml of methanol in place of the extract. The antioxidant activity
(AA) was then calculated using the formula:
AA = [(Ao – Ac)/Ao] x 100,
Where: Ao = absorbance without extract and Ac = absorbance
with extract.
Nitric oxide
Sodium nitroprusside generates nitric oxide in aqueous solution
at Physiological pH, which consequently interacts with oxygen
to produce nitric ions. This was measured by Griess reaction
[10].
Procedure: 3ml of the reaction mixture containing sodium nitroprusside
(10mM) in phosphate buffered saline (PBS) together
with the varying concentrations of the extract (0.5, 0.25, 0.125,
0.0625, 0.003125mg/ml) were incubated in a water bath at room
temperature for 150 minutes. This was followed by the removal of
1.5 ml of the reaction mixture and the addition of 1.5 ml of Griess
reagent. After which, the absorbance of the chromophore formed
was read using spectrophotometer at 546nm. Percentage inhibition
of nitric oxide radical by the extract was calculated using the
formula:
NO = [(1-E/C)] x 100,
Where: C= absorbance value of the fully
Ferric reducing antioxidant power assay (FRAP) assay
The FRAP assay used antioxidants as reductant in a redox
linked colorimetric method with absorbance measured with a
spectrophotometer. A 300mmol/L acetate buffer of pH 3.6 (3.1g of
sodium acetate+16ml of glacial acetic acid made up to 1L with distilled
water, 10mmol/L 2, 4, 6-tri (2-pyridyl 1, 3, 5-triazine, 98%
(sigma-Aldrich) (3.1mg/ml in 40mmol/L HCl) and 20mmol/L of
ferric chloride were mixed together in the ratio of 10:1:1, respectively
to give the FRAP working reagent.
Procedure: A 50μL aliquot of extract was added to 1.5ml of
FRAP reagent in a semi-micro plastic cuvette. Absorbance measurement
was taken at 593nm (A593) exactly 10 minutes after mixing
using 50μL of water as the reference. Thereafter, to standardize
50μL of the standard, iron (III) sulphate, (1mM) was added to
1.5ml of FRAP reagent. All measurement was taken at room temperature
in the absence of light.
Evaluation of total phenolic content
The total phenolic of V.amygdalina extract was determined using
the folin ciocalten assay method of Singleton and Rossi (1965)
[11]. To 0.1ml of 1mg/ml of extract /standard was added 0.9ml of
distilled water. Thereafter, 0.2ml of folic reagent was added. This
was vortex-missed. Subsequently, 1ml of 7 % Na2CO3 solution was
added to the mixture after 5minutes. The solution was followed by
dilution to 2.5ml and then incubated for 90minutes at room temperature.
The absorbance was read at 750nm against the reagent
blank. Standard preparation was carried out by preparing a stock
solution of gallic acid (1mg/ml) aliquots of 0.2,0.4, 0.6,0.8 and 1ml
were taken and made up to a total volume of 2ml.
With the equation as shown below, the total phenolic content
of the plants was then calculated, and expressed as mg gallic acid
equivalent (GAE)/g fresh weight. The analysis was carried out in
triplicates.
Equation (1) - - - - -C=c *v/m
Where: C = total content of phenolic compound in gallic acid
equivalent (GAE); c = concentration of gallic acid established
from the calibration curve, mg/ml; V=volume of extract (ml); m =
Weight of the crude methanolic plant obtained
Evaluation of total flavonoids content
Aluminium chloride colorimetric method described by Zhilen
was used for the determination of the total flavonoidal content of
the plant extract [5]. Water (0.4ml) was added to 0.1ml of extract/
standard, as well as 0.1ml of 5 % sodium nitrite. This was left for
5minutes. Thereafter, 0.1ml of 10 % aluminium chloride and 0.2
ml of sodium hydroxide was added to the solution, and the volume
was adjusted to 2.5ml with water. The absorbance at 510nm was
measured against the blank.
Standard preparation
A stock solution of quercetin (1mg/ml) was prepared. Aliquots
of 0.2, 0.4, 0.6, 0.8, and 1ml were taken and the volume
made up to 2ml with distilled water.
The total flavonoid content of the plant extract was then calculated
as shown in the equation below and expressed as mg
quercetin equivalents per gram of the plant extract. The analysis
was conducted duplicates and mean value considered. X = q×V/w:
Where X= total content of flavonoid compound in quercetin equivalent;
q = concentration of quercetin established from the standard
curve; V=volume of extract (ml); w = weight of the crude
methanolic extract obtained.
Proanthocyanidin content determination
The proanthocyanidin content of the extract was determined
spectrophotometrically [12]. Extracts were diluted to provide a
spectrophometric reading between 0.1 and 0.8 absorbance units.
Procedure: A 0.25ml sample aliquot of adequately diluted
extract was added to 2.25ml of concentrated hydrochloric acid in
n-butanol (10/90, v/v) in a screw top vial. The resulting solution
was mixed for 10 to 15 seconds. Extracts were then heated for
90 minutes in an 85 °C water bath then cooled to 15-25 °C in an
ice bath. The absorbance at 550nm was measured on a UV visible
spectrophotometer. A control solution of each extract was
prepared to account for background absorbance due to pigments
in the extracts. The control solution consisted of the diluted extract
prepared in the hydrochloric acid/n-butanol solvent without
heating.
The proanthocyanidin content was expressed as mg cyaniding
per Kg of sample.
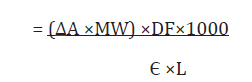
Where:
ΔA = A550sample – A550control
A550 sample = Sample absorbance at 550nm
A550control = control sample absorbance at 550nm
Є = Molar absorbance co efficient of cyanidin (17,360L-1M- 1cm-1)
L= pathlenght (1cm)
MW= Molecular weight of cyaniding (287g/mol)
DF= dilution factor to express as g/L
1000 is the conversion from grams to milligram
Determination of total anthocyanin content
Total anthocyanin content of the extract was determined by
the pH differential method [13].
Procedure: A pH 1.0 buffer solution was prepared by mixing
125ml of 0.2 N KCl with 385 ml of 0.2 N HCl and 490ml of distilled
water. The pH of the buffer was adjusted to pH 1.0 with 0.2 N HCl.A
pH 4.5 buffer solution was prepared by mixing 440ml 0f 1.0 M sodium
acetate with 200ml, 1.0M HCl and 360ml of distilled water.
The pH of the solution was measured and adjusted to pH 4.5 with
1.0 MHCl.
0.5ml of the extract was diluted to12.5ml in the pH 1.0 and
4.5 buffers, and allowed to equilibrate in the dark for 2 hours.
The absorbance of the samples at 512nm (A512nm) and 700nm
(A700nm) was measured on a UV- visible spectrophotometer.
The difference in absorbance (ΔA) between the anthocyanin extract
diluted in pH 1.0 and pH 4.5 buffers was calculated using the
equation below
ΔA= (A512 pH1.0-A700nm pH1.0)-(A512nm pH4.5-A700nm pH 4.5)
The A700nm was employed in the calculation of ΔA to correct
for any background absorbance due to turbidity on the extracts.
The anthocyanin content was expressed as mg cyaninidin 3-glucoside
per 100g berries using a molar absorbance co efficient (Є)
of 26900 L-1M-1cm-1(Guisti and Wrolstad, 2001).
TACY = (ΔA×MW) ×DF×1000
Є ×0.1×1
Where:
TACY= Total anthocyanin expressed as mg cyaniding 3-glucoside/
100g of plant material
MW= molecular weight of cyaniding 3-glucoside (449.2g/L)
DF= dilution factor to expressed the extracts on per gram of
plant basis
Є= molar absorption co efficient of cyaniding 3-glucoside (26900 M-1cm-1)
0.1= is the conversion factor for per 1000 grams to 100 grams basis.
Brine shrimp bioassay
Brine shrimp lethality test was carried out using hatched
Brine shrimp (Artemia salina L) larvae (nauplii) according to the
procedure described by The eggs were hatched in artificial sea
water (16g of sea salt in 50ml of distilled water) by adding 100mg
of brine shrimp eggs to 50ml of sea water that was partitioned
into two compartments. The compartment sprinkled with the
cysts was left dark, while the other compartment was supplied
with bright white fluorescent light. After 24hours of incubation,
the hatched shrimps moved to the illuminated side. Ten brine
shrimps larvae were then counted and transferred to each sample
vial, using a Pasteur pipette and artificial sea water was added to
make 10ml. The sample vials were previously containing solution
of the extract prepared by dissolving 0.2g of the extract in 20ml
distilled water to give concentration of 1mg/ml. The varied concentrations
from the stock solution were transferred to different
graduated container with the aid of a micropipette. The survivors
were counted after 24 hours. Three independent studies were
carried out (n =3).
Statistical Analysis
The results are expressed as mean±SEM using Graph Pad
Prism Graphical-Statistical Package version 5. The difference between
groups was analyzed by Student t-test followed by Dennett’s
test with 5% level of significance (p<0.05).
Results
Antioxidants
The extract was assayed for total content of four major types
of antioxidant properties. The antioxidant constituents were: to tal phenol, total
flavonoid, proanthocyanidins and anthocyanins.
However, the percentage yield of the crude extract used for the
assays is given as 10.11±1.08%. The results showed the total phenolic
content as 1.588±0.04mgGAE/g, which is considerably high
compared to the standard. The total flavonoid content expressed
as quercetin equivalent per gram of the plant extract showed
that the test material had 0.857±0.15mg QUE/g dry weight for
the crude extract (Table 1). These two indices are pointer to an
increased antioxidant activity. The concentration of anthocyanin
in the sample was 0.099±0.08 cyanidin 3-glucoside/100g
for the crude extract, while the concentrations of proanthocyanin
was 0.038±0.05 cyanidin 3-glucoside/100g for the crude
extract. Tannin was also assayed, and it gave a concentration of
1.188±0.04mg/ml (Table 1).

Antiradicals
The result of the antiradical assays carried out on the extract
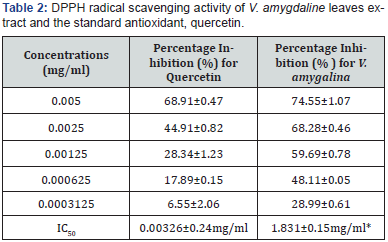
is shown in Table 2. Using the DPPH (2, 2-diphenyl-1-piccrlhydrazyl
hydrate) assay, a well established antiradical assay,
the activity was concentration dependent i.e. activity increases
with increase in concentration. The extract gave the highest inhibition
of 74.55±1.07% at 0.005mg/ml. The calculated IC50 values
for the test extract and standard Quercetin were 1.831±0.15
and 0.00326±0.24mg/ml, respectively Table 2. The extract used
showed activity despite the significant difference (P<0.05) between
the test and standard.
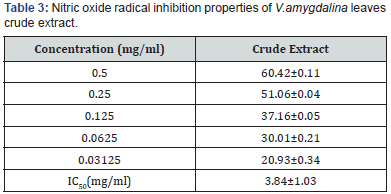
The nitric oxide inhibition assay also showed that V.amygdalina
is a potent scavenger of nitric oxide as shown by the percentage
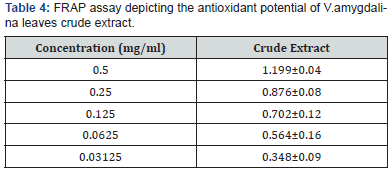
inhibition and IC50 of 3.84±1.03mg/ml Table 3. The FRAP
assay result showed a concentration dependent change when the
FRAP values of the test fractions were determined. Results were
expressed in mmol Fe2+/L. The concentration of Fe2+ in the reaction
mixture at 0.5mg/ml, was given as 1.49±0.18 mmol Fe2+/l for
the test extract (Table 4).
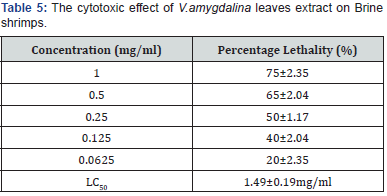
Brine shrimp lethality test
As shown in Table 5, the plant extract showed the highest
percentage lethality to be 75% with LC50 of 1.49mg/ml, whereas,
the LC50 for the positive standard (K2Cr2O7) was found to be
10.91±2.22μg/ml. The plant extract showed concentration at 50%
percentage lethality to be a little greater than 1mg/ml compared
to the standard. In essence, the test sample at the concentration
used could be harmless to the biological system. All values are expressed
as mean±SEM. This result is a triplicate of three independent
experiments.
Discussion
Studies have shown that consumption of biosafe exogenous
and natural antioxidant is beneficial, as regard combating diseas es such as
cancer, arthritis, diabetes, among others. These diseases
emanates from oxidative stress mostly caused by reactive
oxygen species (ROS) [14-16]. Moreover, synthetic antioxidant, including
tert-butylhydroquinone (TBHQ), buthylatedhydroxytoluene
(BHT) and propylgallate have been found to be beneficial, but
toxic, as well as with attendant effects [17,18]. This is shown by
comparing the bio-safe syzygium cumini fruit juice, a natural antioxidant
to the toxic BHT on serum enzymes such as ALT (alanine
transferase), AST (aspartate transferase), alkaline phosphatase
and urea in rats [19]. For this reason, it has become imperative to
continue to investigate and search for more bio-safe antioxidants
that could be relevant in the fight against oxidative stress V. amygdalina
is useful in this regard [20-22]. Kahaliw and his group have
reported on the biosafety of this plant [23]. Moreover, anecdotal
evidence attests to its use in the treatment of different ailments
after boiling, as well as its use in the preparation of soup. This informed
the aqueous extraction carried out, as opposed to the use
of organic solvents, such as methanol and ethanol.
A lot has been reported on V. amygdalina as a functional food.
In order to further establish its biosafety, the result in table 5 and
the work of Kaali justifies V. amydalina as an anti-malaria agent
that is biosafe for all the benefits discoursed above [29]. The study
of Patnaik and Bhatnagar is in agreement with this study [30].
Moreover, Thompson showed comparable results [31] Data from
alcoholic extract of V.amygdalina [32,33] is statistically indistinguishable
compared to this study (Table 5).
Conclusion
On the basis of the data from this current research, V. amygdalina
is a potent antioxidant attributable to their flavanoid and
phenolic constituent that is biosafe for all the health benefits that
is known for.




Comments
Post a Comment