The Prevalence of Bovine Trypanosomiasis in JabiTehnan District of Amhara Regional State, Ethiopia- Juniper Publishers
Juniper Publishers- Journal of Cell Science
Abstract
Cross sectional study was conducted in Jabi Tehnan
District of West Gojjam Administrative Zone of Amhara Regional State,
Ethiopia to determine the prevalence of bovine trypanosomiasis. In the
parasitological survey, blood samples of 164 cattle were examined using a
buffy coat technique. The Packed Cell Volume (PVC) value of each animal
was also measured using hematocrit reader. The overall prevalence of
trypanosomiasis was found to be 15.24% and it consists of 9.76% and
20.73% in Adankegne and Ergib peasants’ association, respectively
(X2=5.783, p=0.056). The most positive cases were due to Trypanosoma congolense (T. congolense ) (80%) followed by Trypanosoma vivax
(T. vivax)(20%). The mean(PCV) values of parasitaemic and aparasitaemic
animals during the study period were 20.75% and 25.07%, respectively.
The variation in mean PCV values were statistically significant
(p=0.01). The study also demonstrated statistically significant
(X2=13.886, p=0.001) variations in prevalence between sexes of cattle,
which were 10.67% and 19.1% in female and male animals, respectively.
The present prevalent study generated valuable information on the
epidemiology of bovine trypanosomosis in the study area and revealed
that trypanosomosis was an important disease affecting the livestock
production
Keywords: PCV; Prevalence; Trypanosoma congolense; Trypanosoma vivax; BovineIntroduction
Livestock is backbone of the socio-economic system of
most of the rural communities of Africa [1]. Ethiopia is known for its
large and diverse livestock resource endowments. Livestock is primarily
kept on small holdings where it provides drought power for crop
production, manure for soil fertility and fuels, serves as a sources of
family diet and sources of cash income (from sale of livestock and
livestock products). Despite large livestock population, Ethiopia fails
to optimally utilize this resource due to different constrains facing
the livestock subsector. Shortage of nutrition, reproductive
insufficiency, management constraints and animal disease are the major
constraints [2]. One of the diseases hampering the livestock subsector
is trypanosomosis [3]. Trypanosomosis is a complex disease of protozoa
that is caused by different species of unicellular parasites
(trypanosome) found in the blood and other tissues of vertebrates,
including livestock, wild life and people [4]. Trypanosomosis limited to
the extension of natural herds particularly in Africa were the presence
of the tsetse fly density access to woodland and savanna areas with
good grazing potential [3]. It is a serious constraint to agricultural
production in extensive areas of the tsetse infested regions which
accounts over 10 million squares of the tropical Africa [5].Ethiopia is
one of the countries suffering from the impact of trypanosomosis. In
Ethiopia, it is estimated that some 10 to 14 million heads of cattle and
an equivalent number of small ruminants together with a significant
number of equines and camels, are exposed to the risk of trypanosomosis
[6]. Six species of trypanosomes are recorded in Ethiopia and the most
important trypanosomes in terms of economic loss in domestic livestock
are the tsetse transmitted species T. congolense, T. vivax and T. brucei [3].
Tsetse flies in Ethiopia are confined to western and
south-western parts of the country between 33°C and 38°C E longitude and
5°C and 12°C N latitude. It is estimated to cover an area of 140, 000,
220, 000 km2[7]. Tsetse infested areas follow the major river systems;
namely, Abay (Blue Nile), Baro, Akobo, Didessa, Ghibe and Omo river
systems [8]. Five species of Glossina (Glossina morsitans submorsitans, G. pallidipes, G. tachinozdes, G.f. fuscipes and G. longipennis)
have been recorded in Ethiopia [3]. According to National Tsetse and
Trypanosomosis Investigation and Control Center [7], tsetse transmitted
animal trypanosomosis still remains as one of the largest causes of
livestock production losses in Ethiopia. The effects of trypanosomosis
is not only the direct losses resulting from mortality, morbidity,
infertility of the infested animals and costs of controlling the
disease, but also
due to indirect losses, which include exclusion of livestock and
animal power-based crop production from the huge fertile tsetse
infected areas. Annual estimated losses for Ethiopia as a result
of trypanosomosis is roughly $200 million, in terms of mortality
and morbidity losses in livestock (excluding utilization of fertile
land for crop and livestock production) and the costs included in
controlling the disease [9].
The most prevalent trypanosome species in tsetse infested
areas of Ethiopia are T. congolense and T. vivax. Rowlands et al.
[10] reported a prevalence of 37% for T. congolense in Southeastern
Ethiopia. Abebe and Jobre [11] reported an infection
rate of 58% for T. congolense , 31.2% for T. vivaxand 3.5 % for T.
bruceiin Southern Ethiopia. In the same report it is also indicated
that 8.71% infection rate was recorded in the highlands (tsetse
free areas) of which 99% is due to T. vivax. Different workers [12-
14] indicated a prevalence of 17.2%, 21% and 12 % in Metekel
district, in upper Didesa Valley and Southern Rift valley areas
of tsetse transmitted regions, respectively, and the dominant
species was T. congolense .
In the western part of Amhara Regional State bordering
the Abay river basin, one of the north western tsetse belt areas
of Ethiopia, tsetse transmitted trypanosomes are becoming a
serious threat for livestock production and agricultural activity in
particular. Reports made by the Regional Veterinary Laboratory
in 1999 indicated the presence of tsetse fly transmitted
trypanosomosis in three districts of the region (Bure, Jabi Tehnan,
and Ankesha) bordering the Abay valley areas. A preliminary
survey conducted in Dembecha district by the Ethiopian Science
and Technology Commission and West Gojjam Veterinary Office
in 2001 indicated a trypanosome infection rate of 23% with a
dominant species of T. congolense and tsetse fly identified was
G. morsitans. Therefore, this study was undertaken to determine
the prevalence of bovine trypanosomosis, to identify the
dominant species of trypanosomes involved, and to assess the
PCV values of cattle in relation to the risk factors associated with
the disease.
Materials and Methods
Study area
The study was conducted in Jabi Tehnan district of west
Gojjam Administrative Zone of Amhara Regional State. The
district covers an area of 112,772.1 ha and bordered by Quarit
and DegaDamot in East, Burie in West, Sekela in North, and
Dembecha and Abay River in the South. The annual mean
temperature for most part of the district is 14-32°C and the
elevation varies from 1500-2300 mm above sea level (m a. s. 1)
with mean annual rain fall of 1250mm. The livestock populations
that are found in Jabi Tehnan district include cattle, sheep, goats,
horses, mule, donkey and poultry. Among these animals, cattle
are the dominant species raised in the area. The cattle population
in the district is estimated to be about 187,481[15] (Figure 1).
Study animals
The study was conducted on local Zebu cattle. These animals
were raised in different villages of Adankegne and Ergib of Jabi
Tehnan district. The animals examined in this particular study
were representing different Kebeles. Sex and body conditions of
cattle were also being recorded accordingly.
Study design
The retrospective data of cross sectional survey was
conducted to determine the prevalence of bovine trypanosomosis.
The two sites were selected based on their higher prevalence of
trypanosomosis than any other Kebeles of Jabi Tehnan district.
Sample size and sampling methods
The sample size was calculated using previous prevalence of
11.7% by [17] and desired absolute precision of 5% as per the
standard procedure described by Thrusfield [18] shown below.
An estimated minimum sample size of 159 cattle was obtained;
however, we were able to examine 164 cattle for our study.

Study Method and Procedure
Buffy coat technique
Blood was collected from an ear vein using heparinized microhematocrit
capillary tube and the tube was sealed. A heparinized
capillary tube containing blood was centrifuged for 5 minutes
at 12,000rpm. After centrifugation, trypanosomes were usually
found in or just above the buffy coat layer. The capillary tube
was out using a diamond tipped pen 1mm below the buffy coat
to include the upper most layers of the red blood cells and 3mm
above to include the plasma. The content of the capillary tube
was expressed on to slide, homogenized on to a clean glass slide
and covered with cover slip. The slide was examined under x40
objectives and x10 eye piece for the movement of parasite [19].
Measuring of packed cell volume (PCP)
Blood samples were obtained by puncturing the marginal
ear vein with a lancet and collected directly into a capillary tube.
The capillary tubes were placed in micro-hematocrit centrifuge
with sealed end outer most. The tube was loaded symmetrically
to ensure good balance. After screwing the rotary cover and
closing the centrifuge lid, the specimens were allowed to revolve
at 12,00rpm for 5 minutes [4,20]. Tubes were then placed in
hematocrit and the readings were expressed as a percentage of
packed red cells to the total volume of whole blood. Animals with
PCV ≤ 24% were considered to be anemic [21].
Data analysis
Row data on individual animals and parasitological
examination results were inserted into MS Excel spread sheets
to create a data-base. Students t-test were employed to compare
between the two-independent mean PCV values of animals from
an individual site (peasant’s association). Chi-square test was
also employed to assess the association between the risk factors
and the disease. While analyzing data, p-values (p)<0.05 were
registered as statistically significant. Otherwise, recorded as
insignificant.
Result
Prevalence
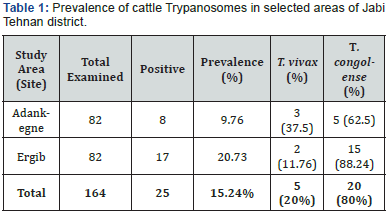
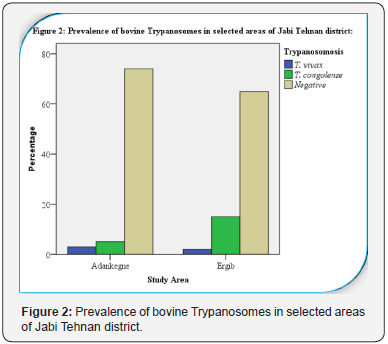
Out of the total 164 (75 females and 89 males) cattle
examined, 25 (15.24%) were found positive to trypanosomosis.
The prevalence varied between different study areas, in which
9.76% (n = 8) and 20.73% (n = 17) were recorded at Adankegne
and Ergib peasant’s association, respectively. The variation in the
prevalence of bovine trypanosomosis between the study sites
were not statistically significant (X2= 5.783; p = 0.056) (Table
1 and Figure 2). The most prevalent trypanosome species in the
study area was T. congolense (80%) followed by T. vivax(20%)
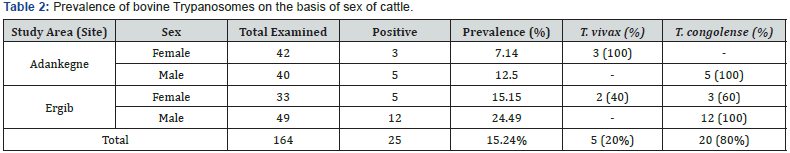
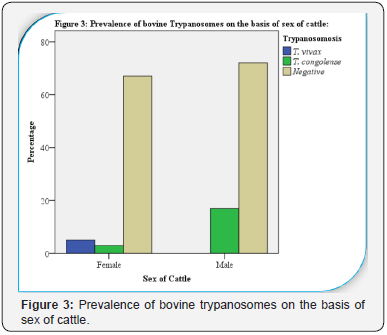
(Table l and Figure 2). The prevalence of bovine trypanosomosis showed statistically significance difference between sexes of
cattle, in which, higher in male animals (19.1%) as compared to
females (10.67%) (X2= 13.886; p = 0.001) (Table 2 and Figure 3).
Hematological findings
Discussion
The study revealed that the prevalence of bovine trypanosomosis
in the area was 15.24% (25/164) which was higher compared
with the previous findings of Bitew et al. [17] in the same
area (11.7%). The difference in prevalence might be due the site
from which the blood samples were collected. However, there
were tsetse control intervention, and continuous treatment of
sick animals as well as deforestation for the cultivation of land.
These activities could have led to the reduction of tsetse fly population
along with the decline of tsetse borne trypanosomosis
in the study area. But the continuous and longtime utilization of
trypanocidal drugs particularly Diminazin aceturate in the study
area contribute for the development of drug resistance, so that
the prevalence of trypanosomosis was higher than the previous
finding due to the above reasons.
In this study, two species of trypanosomes; namely, T.
congolense and T. vivax were retrieved from inspected cattle.
Majority of infections were also due to T. congolense. The higher
proportion of T. congolense infection in the study area was in
agreement with trypanosome species prevalence data from
other tsetse infested region of Ethiopia where T. congolense is
the most prevalent species in cattle [11]. In the same report it
was also indicated that in tsetse free area of highlands, 99% of
prevalence was due to T. vivax [12-14]. But in this study area, the
prevalence of T. vivaxwas less than T. congolense in both peasant
associations because the two sites are located adjacent to tsetse
infested belts. Leak [22] and Degneh et al. [23] also indicated that
T. vivax was highly susceptible to treatment while the problems
of drug resistance were higher in T. congolenseM.
In the current study, higher infection rate of trypanosomosis
was detected in males (19.1%) as compared to in female cattle
(10.67%) with statistically significant difference (X2= 13.886; p
= 0.001). Different researchers work supported this finding [22-
25]. Although the variation was not statistically significant, Yalew
and Fantahun [26], and Teferi and Biniam [27] had also reported
higher prevalence of bovine trypanosomes in males than in
females (X2 = 0.85, p=0.35 and X2= 0.10, p>0.05, respectively).
According to Gemtessa and Dera [28], the higher prevalence of
trypanosomes in males rather than in females might be related
to the hardworking of male animals. Similarly, the variation in
the prevalence between the two sexes might also be associated
with that male animals travel longer distances to tsetse abundant
areas for draught and ploughing purposes, and the journey
creates stress leading to susceptibility to the infection [23,)].In
contrast to this study,Kitila et al. [30] at Yayo District Illuababora
Zone of Western Oromia and Tamirat et al. [31] at Enemorena
Ener Woreda of Gurage Zone were found higher prevalence of
bovine trypanosomosis in female cattle than males.
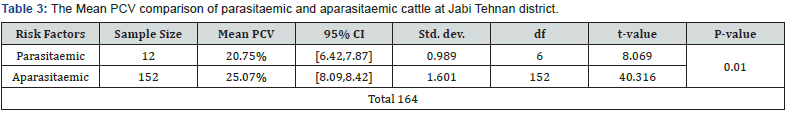
Comparing the mean PCV values of cattle, significantly
(p=0.01) low PCV was recorded in parasitaemic animals (25.07%)
(SD = 0.989; df = 6; t-value = 8.069) than in aparasitaemic
animals (20.75%) (SD = 1.601; df = 152; t-value = 40.316). This
finding was in line with previous works conducted at different
regions of Ethiopia by many authors [22,25]. In the absence of
other diseases causing anemia, a low PCV value of individual
animals is a good indicator of trypanosome infection [23,32].
Trypanosomosis might adversely lower the PCV values of
infected animals [33]. A survey conducted in cattle in Hawagelan
District of West Wellega Zone [34] revealed that the mean PCV of
trypanosome infected animals was significantly lower (20.8±3.2
%) compared to non-infected animals (24.9±3.8 %). A later study
in Northwest Ethiopia [35] in cattle experimentally infected with
T. vivaxi solates also showed that the mean PCV, Hb and total RBC
count were lower (p < 0.001) in all infected groups than in noninfected
control animals. In Nigeria, domestic ruminants that
were naturally infected with trypanosomes had significantly
lower (p<0.05) PCV and RBC counts compared to uninfected
animals [36]. Lower herd average PCVs for trypanosomepositive
cattle compared to trypanosome-negative cattle have
also been reported from Ghana [37], Zambia [32], Cameroon
[38] and Gabon [39].
In spite of the fact that trypanosome infection has significant
association with risk factors such as age and body condition
scoring, as reported by many scholars, this study had not
demonstrated and regarded as limitations.
Conclusion
From this study it is possible to conclude that trypanosomosis
is an important disease and a potential threat affecting the health
and productivity of cattle. The major species of trypanosomes
in the study area were T. congolense and T. vivax. To sum up,
infection with trypanosomosis negatively affects PCV and
body condition of animals. This indicated that trypanosome
infection of cattle causes loss of body weight and production.
Trypanosomosis control measures should be targeted on
tsetse fly destruction and control methods such as pour-on
and effective trypanocidal drug applications. Similarly, rearing
or raising of trypanosomosis resistance cattle breeds is now a
day in practical. Otherwise, the problems will increase through
the aide of global warming. In conclusion, further study on the
occurrence of tsetse and trypanosomosis at different season of
the year at different altitudes and species of animals should be
conducted.
Acknowledgement
The Authors would like to thank Bahir Dar Regional
Veterinary Diagnostic and Investigation Center for logistic
and material support in the realization of this study. And we
would also to adders our thanks to Dr. HabtamuTamrat for his
constructive comments and editing the entire work.
Conflict of Interest
Authors declare that no competing of interests in the
publication of this work.




Comments
Post a Comment