Plasma Membrane H+-ATPase during Embryo Dormancy and Dormancy Release in Horse Chestnut Seeds- Juniper Publishers
Juniper Publishers- Journal of Cell Science
Abstract
Horse chestnut seeds harvested in October were
exposed to wet cold stratification for 14 weeks, thus providing a way
for seeds to overcome the state of deep physiological dormancy and
acquire the ability to germinate. During first 5-6 weeks, a small
portion of seeds germinated hardly and slowly while later on seeds
germinated more uniformly and rapidly. The experiments with facilitated
water supply have shown that 5-6 weeks is the state of embryo dormancy,
which did not depend on water inflow, whereas during 6-14 weeks these
embryos became capable to germinate, but this capacity was limited by
water penetration through seed coat. Two distinct periods were
identified in deep dormancy, namely embryo dormancy and coat-imposed
dormancy (dormancy release). They differ in plasma membrane H+-ATPase
behavior. During embryo dormancy, the the transformation occurred from
autoinhibited form preformed during seed maturation to an active form,
whereas during dormancy release the activity of enzyme increased up to
the levels typical for germinating seeds.
Keywords: Seed; Embryo dormancy; Dormancy release; Seed germination; Plasma membrane H+-ATPase
Introduction
Plasma membrane H+-ATPase is a unique membrane enzyme capable to transport H+ ions from cytoplasm to cell walls by exchanging H+ ions for K+
ions. It can fulfill a key role in seed germination, particularly in
the beginning of cell elongation in the axial organs of seed embryo [1]. The mechanism of its action is the following: H+
ions in cell walls cause acidification favoring the cell wall loosening
resulting in their high extensibility. Under the pressure of entering
water, these cell walls extend that vafors the beginning of cell
elongation. This is the way how plasma membrane H+-ATPase participates in seed germination providing H+ ions extrusion prior to seed germination [2].
Our experiments are usually performed with the seeds of horse chestnut Aesculus hippocastanum
distinguished by their germination occurring only by cell elongation;
cell division starts in their roots at the length of 3cm [3]. These seeds easily germinate in warm countries with humid climate [4].
However, in Russia with its cold winter, these seeds after shedding
enter deep dormancy to germinate in spring. In this paper, the behavior
of plasma membrane H+-ATPase was studied in such dormant and starting germination horse chestnut seeds.
Materials and Methods
Horse chestnut seeds were collected in the Main
Botanical Garden of Russian Academy of sciences, in Moscow, Russia.After
seed extraction from fruit covers, they were uniformly distributed in
the boxes filled with wet sand and placed into cold room at 4 OC.
Such treatment called "cold wet stratification" imitates the natural
conditions for shed seeds inside the litter, but controls the
environmental conditions.
The seeds were regularly taken from the boxes, washed
and used for analyses. Some seeds were incubated in water at 27 °C
being intact, while in another portion of seeds the "windows" in seed
coat were made, just above the embryo axis. This treatment facilitated
water penetration to the embryo. Such seeds with "windows" were also
incubated in water under the same conditions. In both seed portions,
intact and with "windows", the rate of radical protrusion (growth
initiation) was regularly recorded.
In another series of experiments, every three weeks
the axial organs of the embryos (embryo axis) were isolated, weighed and
transferred to Petri dishes with distilled water or 10-6M fusicoccin, an activator and stabilizer of plasma membrane H+-ATPase [5]. After incubation at 27 OC,
axial organs were weighed and used for acidification measurements.
Twenty axes were preliminary incubated for some minutes in10-3M KCl and transferred to pH-meter cell filled with 10-4M KCl . H+
ions transferred by the enzyme from axial organs into cell walls
(apoplast) were washed out into the ambient solution, their accumulation
was recorded by pH shift from pH 6.2.-6.5 (optimum pH for this enzyme)
to acid values during 10 minutes.
Results
Horse chestnut seeds enter in autumn the deep
physiological dormancy for 14 weeks in average. During first 5-6 weeks,
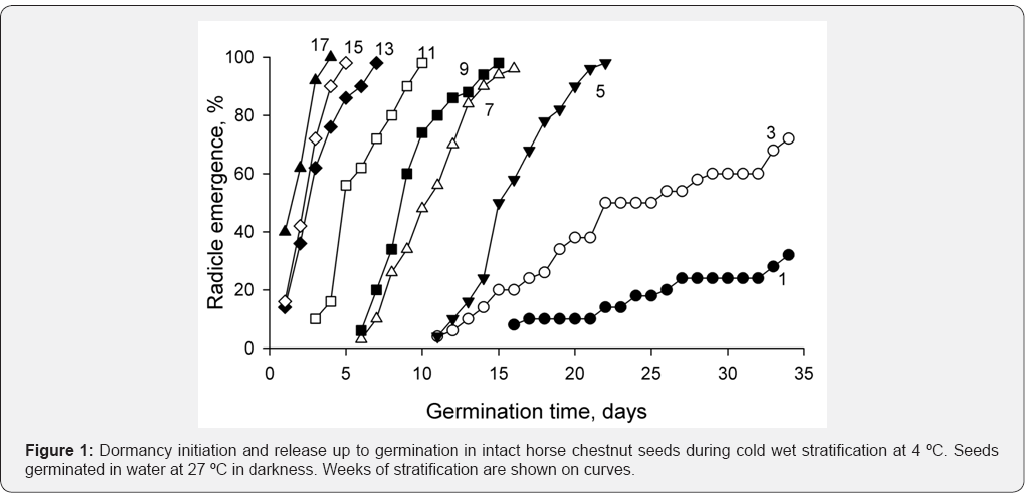
seeds germinated very slowly and percent of germinating seeds was low (Figure 1).
Later on percent of germinating seeds increased and rate of germination
increased too. By week 14, practically all seeds were capable to
germinate and germination time was 1-2 days in average. The seeds with
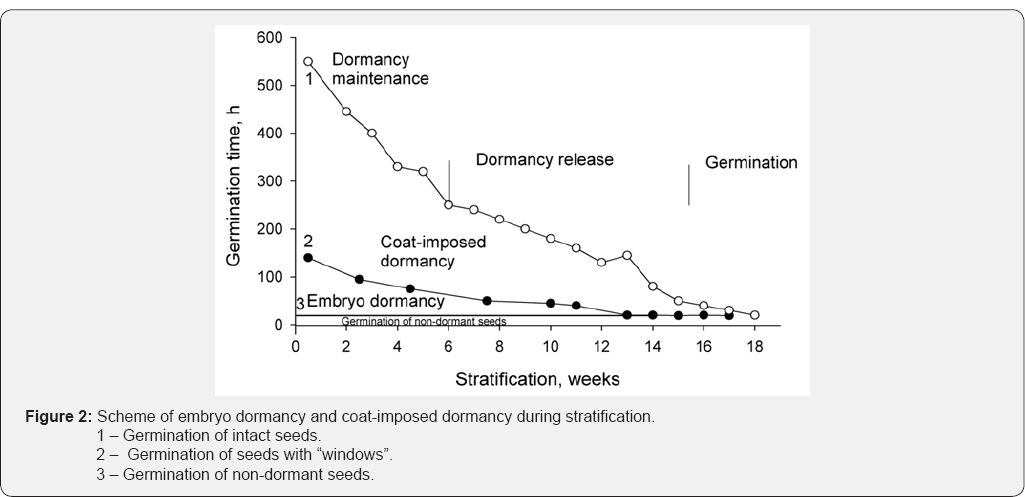
the "windows" over axial organs of embryo were under more favorable
conditions, they absorbed water through these "windows" at higher rate
as compared to intact seeds. For this reason, the seeds with "windows"
germinated earlier (Figure 2, curve 2) than intact seeds (Figure 2,
curve 1).The germination rate in hours was calculated for each point on
both curves, and used as the ordinate axis. The area plot between
curves 1 and 2 indicates the dependence of germination on water supply,
whereas the area plot between curve 2 and curve 3 (germination rate of
non-dormant seeds) indicates the germination dependence on the dormancy
itself. These observations permitted us to distinguish the dormancy of
embryo (first 5-6 weeks) from the coat-imposed dormancy manifesting
itself during 6-14 weeks and depending on water penetration through seed
coat.
The next aim of the work was the measurement of axial
organ capacity to acidify the ambient solution and to verify whether
the increase in acidification is sensitive to fusicoccin. The
acidification of solution and effect of fusicoccin were measured every 3
weeks of stratification in axial organs from non-emerged seeds in wet
sand, and then in axial organs of seeds gradually stratified in wet sand
up to radicle protrusion (Figure 3).
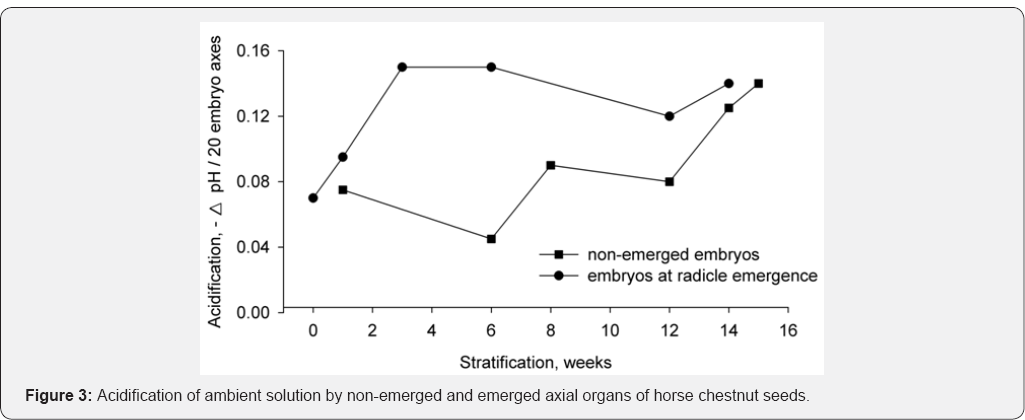
Another series of measurements was carried out with
seeds achieving the radicle emergence (growth initiation) state. For the
period of embryo dormancy (up to 6 weeks) very weak acidification was
recorded, but later on, during the period of coat-imposed dormancy, rate
of acidification increased (lower curve) up to the level typical of
radicle protrusion. This level corresponds to axial organs at radicle
emergence (upper curve).Therefore, the activation of acidification
occurred in seeds during dormancy release. However, the acidification
during embryo dormancy did not respond to the incubation in fusicoccin (Figure 4),
whereas the activation of acidification during dormancy release
responded by stimulation of acidification. This fact clearly indicates
that activation of acidification is due to the activation of plasma
membrane H+-ATPase.
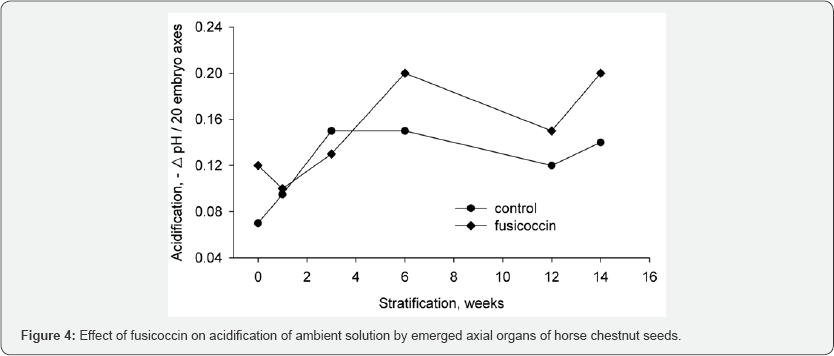
Discussion
The above data demonstrate the following results:
1. Embryo dormancy is characterized by low acidification, not stimulated by fusicoccin,
2. Dormancy release is the period of plasma membrane H+-ATPase activation, as follows from additional activation of the enzyme by fusicoccin.
The first observation can be explained by the
transformation of enzyme molecule from autoinhibited to active
state.autoinhibitory domain is located in C terminus of the regulatory
domain [6].
Phosphorylation of the penultimate threonine residue induces the
binding of regulatory 14-3-3 proteins to the C terminus. This
interaction is believed to cause a detachment of the autoinhibitory
domain. This release of the regulatory domain goes along with a
transformation of the enzyme from the low activity state to the high
activity state [7].
According to recent observations, N terminus of the enzyme also
participates in activation of regulatory domain; the N and C termini are
constituting together the autoinhibitory regulatory module. It is
tempting to suppose that by its nature embryo dormancy is an
autoinhibitory state of the plasma membrane H+-ATPase and that dormancy release of seed embryo is due to the enzyme transition from autoinhibited state to active state.
As to further activation of enzyme activity during
dormancy release, we were unsuccessful to detect expected enhancement of
phosphorylation of penultimate threonine in axial organs of horse
chestnut seeds. It is quite possible that phosphorylation- independent
interaction takes place between 14-3-3 protein and the plasma membrane H+-ATPase [8]. It is supposed that 14-3-3 proteins react with phosphothreonine946
at the very end of C domain. Phosphorylation-independent binding of
14-3-3 protein with this motive is induced by fusicoccin, which binds to
the (14-3-3 + H+--ATPase) receptor. The presence of 14-3-3 protein side by side with H+-ATPase and endogenous fusicoccin legends was demonstrated earlier [1].




Comments
Post a Comment