Development and Evaluation of Antidiabetic Potential of Polyherbal Formulation in Streptozotocin Induced Animal Model- Juniper Publishers
Juniper Publishers- Journal of cell science
Abstract
Background: Diabetes mellitus (DM) is a group
of disorders that results in too much sugar in the blood due to
impairment of lipids, carbohydrates, proteins metabolism.
Aim and objectives: Development and Evaluation
of Polyherbal formulation (PHF) and determination of antidiabetic
potential of developed formulation in Streptozotocin induced animal
model
Method: In the present study plant parts Azadirechta indica (AI) leaves, Moringa Oleifera (MO) fruits and Andrographis paniculata
(AP) root and stem were collected and evaluated as per physico-chemical
parameters and active chemical constituents were extracted using
hydroalcoholic solvent. The active compounds present in all the three
extracts were identified by preliminary phytochemical screening. PHF was
prepared in a ratio of 1:1:1 quality of the finished product was
evaluated on the parameter’s angle of repose, loose bulk density, tapped
bulk density, carr’s index and hausner ratio as per the World Health
Organization’s (WHO) guidelines for the quality control of herbal
materials. The acute toxicity study of PHF were performed as per OECD
guideline 423, rats were orally administered 250, 500, 1000 and
2000mg/kg over 14 days. The oral glucose tolerance test (OGTT) was
performed at 200 and 400mg/kg body weight. Antidiabetic activity of the
PHF (200 and 400mg/kg) was screened against streptozotocin (STZ) induced
diabetes in rats and glibenclamide was used (5.0mg/kg body weight) as
standard drug. The investigational drug was administered for 14 days and
the effect of the PHF on blood glucose levels was studied at 14th day
after interventional period. At the end of the study, the blood samples
were collected from all the animals for biochemical estimation.
Result: The plant parts AI leaves, MO fruits,
AP stem and leaves were evaluated as per physicochemical parameters and
they were found as per API. Preliminary phytochemical screening of
hydroalcoholic extracts were revealed that presence of alkaloids,
glycosides, saponins, flavonoids, carbohydrates, steroids, tannins and
phenolic compounds in each extract. PHF were developed by mixing of each
extract in the same ratio and evaluated. It was found to be angle of
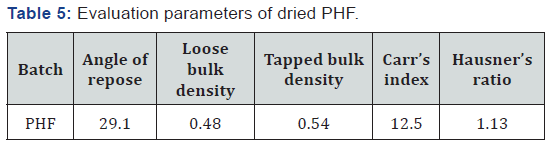
repose (θ) 29.1, loose bulk density 0.48gm/ml, tapped density 0.54gm/ml,
carrs index 12.50%, hausner’s ratio 1.13. Diabetes was induced by STZ
and treated with PHF did not show any change in behavior and no
mortality was observed during interventional period upto the dose level
2000mg/kg. OGTT was performed by oral administration of PHF with dose
200 and 400mg/kg body weight result was found to be gradually decreased
in blood glucose level 75.75±1.92mg/dl and 72±2.73mg/dl at 180min from
the study it was predicted that PHF possess Anti-hyperglycemic activity.
Experimental study was shows that on repeated administration of PHF and
glibenclamide for 14 days, a sustained and significant decrease in the
average blood glucose level of diabetic rats was observed. End of the
interventional period biochemical parameters were studied, and it was
found to be level of SGOT and urea level remain constant at dose of
200mg/kg, decrease in SGPT is near to standard and decrease in
creatinine level is greater than Std at dose of 400mg/kg.
Conclusion: PHF containing extracts of (Azadirecta indica, Moringa oeifera and Andrographis paniculata)
showed significant antidiabetic and antihyperlipidemic activity which
was close to standard drug. Along with remarkable reduction in Total
Cholesterol (TC) level and increased in High Density Lipoprotein (HDL)
STZ induced diabetes rats. The formulation has emerged as potential
combination which can challenge the synthetic drug.
Keywords: Diabetes mellitus; Azadirechta indica; Moringa oleifera; Andrographis paniculate; Polyherbal formulation, Glibenclamide
Introduction
Importance of herbal in mankind
Herbal drugs play an important role in the
development of potent therapeutic agents. Furthermore, it has proven
their potential for the prevention of several ailments. Earlier human
beings started their studies on diseases and its treatments, but there
was no evidence found that people have prehistoric use of synthetic
medicines for their sickness [1]. However, they struggled to make use of
the things, which could easily procure. The most common thing was found
in their surrounding was plants and animals. Several plants were found
suitable as a
food supplement; some were poisonous and have medicinal
importance [2]. Keeping this information in consideration,
herbs were transferred from their origin to generation as folk
medicine. So, the herbal medicine was known from ancient
times. This is only because of the belief that many herbal
medicines are known to be free from side effects. Furthermore,
it is fact that the discovery of the new synthetic drug is time
consuming & an expensive. In the present scenario, the demand
for herbal products is growing exponentially. All over the world
pharmaceutical companies are currently conducting extensive
research on plant materials for their probable medicinal
value [3]. Research needs in the field of herbal medicines are
enormous; the identification of active compounds from the
plants source is still remaining a challenge. So, there should be
research-based confirmation on either whole herbs or extracted
compounds are superior. The issue of herb–herb and herb–drug
interactions is also an important issue, which requires increased
awareness and study, as polypharmacy and polyherbacy are
common. The new technologies, such as nanotechnology and
novel emulsification methods are used in the formulation of
herbal products, which mainly affect bioavailability and the
efficacy of herbal components and this also needs study. This can
lead to reinvestigation of some agents that failed earlier trials
and can be restudied and redesigned using new technologies to
determine whether they can be modified for better efficacy and
fewer side effects [4]. Today, there is an urgent need to develop
safer drugs for the treatment of various disorders. As a result,
there is a growing interest in the pharmacological evaluation of
various plants used in traditional systems of medicine [5].
Diabetes mellitus
Diabetes Mellitus (DM) is a metabolic disorder associated
by impairment in the metabolism of carbohydrate, fat and
proteins which was recognized by insufficient insulin secretion
or mounting resistance to its action [6]. DM develops due
to obesity which is also an increasing problem worldwide,
Induces atherosclerosis and other metabolic syndromes [6-
9]. According to the requirements of insulin DM was classified
into two main categories; insulin dependent diabetes mellitus
(Type 1), and non-insulin dependent diabetes mellitus (Type 2)
[10]. Which were proposed by WHO in 1980 and 1985 changed
new classification system were identified four types of diabetes
mellitus, Type 1 insulin dependent diabetes mellitus, Type 2
non-insulin dependent diabetes mellitus and Type 3 is Maturity
Onset Diabetes of the Young (MODY) as well as Gestational
Diabetes Mellitus (GDM) was classified as Type 4 [11].
Materials and Methods
Drug and chemicals used
Glibenclamide (USV Pharma Ltd. India), Straptozotocin (Lab
chemicals, India), one touch glucometer (Johnson & Johnson,
India), Ethanol (Qualigens, India) and other chemicals were
used of analytical grade.
Collection, identification and authentification of plant materials
In the present study, the fresh leaves of Azadirechta
indica, fruits of Moringa oleifera and fresh leaves and roots
of Andrographis paniculata were collected in febuary, 2018,
from Raipur, Chhattisgarh, India. The plants were identified
and authenticated by Dr. S. Prakash Rao, Department of
Phytochemistry and Pharmacognosy, Columbia Institute of
Pharmacy, Raipur, Chhattisgarh, India.
Quality assessment/Physiochemical evaluation of plant
materials
Each plant parts were crushed and converted into fine
powders than quality assessment of plant materials was done as
per the standard procedure of Ayurvedic Pharmacopeia of India.
Different parameters were tested with the methods describe in
API.
a. Foreign organic matter: According to Ayurvedic
Pharmacopeia of India, Foreign matter is described as any
material that consist of part of organ or organ part from
which the drug is derived. The plant should be free from
any foreign particle like dust, insects, faecal matter etc. The
percentage of foreign matter should not be more than the
limit prescribed in monograph. There should not be any
contamination in drug material used for developing the
polyherbal formulation (PHF).
b. Procedure: 100-500gm of plant materials were
weighed and spread as a thin layer and was inspected first
with naked eyes and then with the use of lens (6x). All the
foreign matter was
c. Separated, weighed and percentage was calculated.
d. Determination of total ash value: 3gm of dried
powered sample was weighed in silica dish and it was
incinerated at a temperature not exceeding 450 °C until it
gets free from carbon. The incinerated material was cooled,
weighed and percentage of ash was calculated with reference
to air dried drug.
e. Determination of acid insoluble ash value: Ash
obtained was boiled with 25ml of dil. HCL for 5 minutes
filtered and insoluble matter was collected in crucible and
washed with hot water and ignited till constant weight. The
percentage of acid insoluble ash was calculated with respect
to air dried drug.
f. Determination of alcohol soluble extractive value:
5gm of powdered drug was macerated with 100 ml of alcohol
in cork fitted conical flask. Solution was shaken frequently
for 6hrs. and was allowed to stand for 18hrs. After 18hr.
content was filtered and 25ml of filtrate was evaporated to
dryness in a shallow dish at 105 °C to constant weight and
percentage of alcohol soluble extractives was calculated
with reference to air dried drug.
g. Determination of water-soluble extractives: 5gm
of powdered drug was macerated with 100ml of water in
cork fitted conical flask. Solution was shaken frequently for
6hrs and allowed to stand for 18hrs. After 18hr. content was
filtered, and 25ml of filtrate was evaporated to dryness in
a shallow dish at 105 °C to constant weight and percentage
of water soluble extractives was calculated with reference
to air dried drug. The data generated in respect of above
findings will be used as in-house standards.
Preparation of hydro-alcoholic (HA) extracts
The plant parts were washed, shade dried and powdered.
In order to prepare the PHF, about 500gm of Azadirecta Indica
(leaves), 500gm of Moringa Oleifera (fruits) and 500gm of
Andrographis paniculata (roots and leaves) powders were
soaked overnight separately in 1000-1200ml of Petroleum Ether
(PE). After 3 days the suspension was filtered and PE was to be
evaporated overnight. Again, the dried powders were separately
resuspended in a Stoppered container with the HA solvent.
Allowed to stand at room temperature for a period of 7days.
Additionally, extract was concentrated to dryness in a rotary
evaporator (Buchi type) under reduced pressure and controlled
temperature (37-40 °C) to get percentage yield.
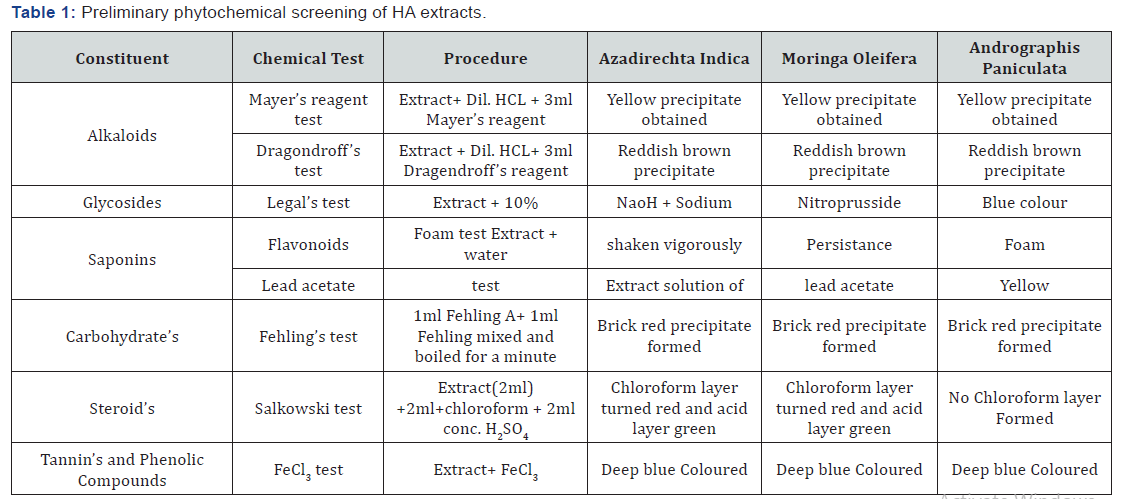
Preliminary phytochemical screening of HA extracts:
Crude extract of plants was subjected to different chemical tests
to detect the presence of various phytochemical constituents as
per procedure adopted in literature by Madhav and Saha. The
details are incorporated below in the following Table 1. Results
of the entire chemical test are discussed in Results.
Design and development of PHF
From the extracts of three plants Azadirechta indica (leaves),
Moringa Oleifera (fruits) and Andrographis paniculata (roots and
leaves), formulation have been made by blending the extracts in
ration 1:1:1.
Evaluation of polyherbal formulations
Prepared PHF was evaluated on following parameters:
a. Angle of repose
Angle of repose was determined by using funnel method.
The accurately weighed blend was taken in a funnel. The height
of the funnel was adjusted in such a way that the tip of the funnel
just touches the apex of the heap or head of blend. The drug
excipient blend was allowed to flow through the funnel freely on
to the surface. The diameter of the powder cone was measured,
and angle of repose was calculated using the following equation:
tan θ = h/r
Where, h = height of powder cone formed, r = radius of the
powder cone formed
b. Loose bulk density
Apparent bulk density was determined by pouring a weighed
quantity of blend into graduated
cylinder and measuring the volume and weight.
LBD = Weight of the powder/volume of the packing
c. Tapped bulk density
It was determined by placing a graduated cylinder, containing
a known mass of drug excipient blend. The cylinder was allowed
to fall under its own weight on to a hard surface from the height
of 10cm at two second intervals. The tapping was continued
until no further change in volume was noted.
TBD = Weight of the powder/vol of the tapped packing
d. Compressibility index
The Compressibility index of the blends was determined by
Carr’s compressibility index.
Compressibility index (%) = (TBD-LBD) x 100/TBD
e. Hausner ratio
It is the measurement of frictional resistance of drug and
ideal range should be 1.2-1.5. It is determined by using the
following formula:
Hausner ratio= TBD / LBD
Acute toxicity study of PHF as per OECD guidelines
Preparation of formulations: For dosing 100ml of each
formulation was prepared by dissolving 5gm of formulation in
100ml of distilled water (so, 1ml contain 50mg of drug).
Experimental animals: Adult Wistar rats (180±10g) of
either sex were obtained from Columbia institute of pharmacy,
Raipur, Chhattisgarh, india. The animals were housed in large,
spacious polyacrylic cages at an ambient room temperature
with 12h light/12h dark cycle. Rats had free access to water and
rodent pellets diet (Hindustan Lever Ltd, Bangalore, India). The
study was approved by the Institute Animal Ethics Committee
and all the animal experiments were carried out according to
the Committee for the Purpose of Control and Supervision of
Experiments on Animals (CPCSEA) guidelines, Regd. No. 1321/
PO/ReBi/S/10/10/CPCSEA.
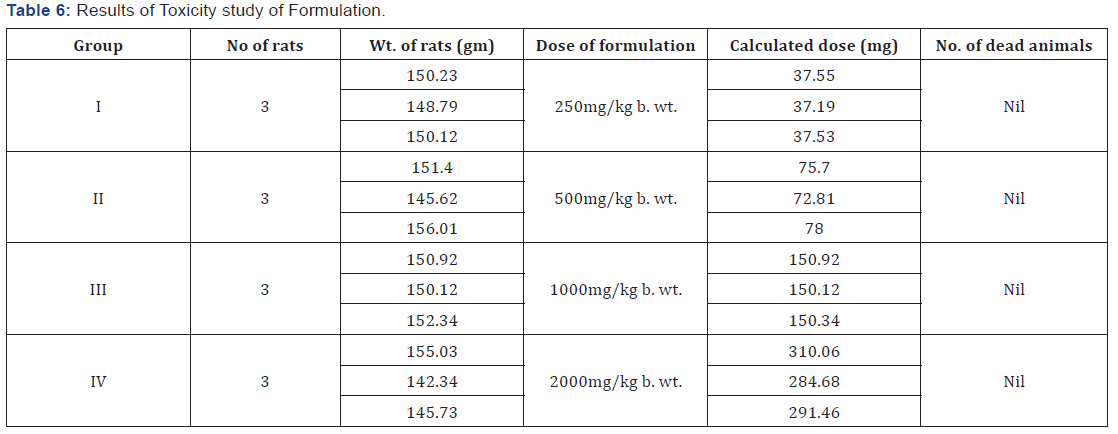
Acute toxicity study of PHF: Acute toxicity studies were
carried out in adult female albino rats weighing between 130-
180gm by Acute Oral Toxicity method of OECD Guideline No 423.
They were administered (orally) with varying doses (250, 500,
1000 and 2000mg/kg body weight) for each of six formulations.
Animals were divided into 5 groups of three animals each and
were acclimatized for 5 days. Prior to dosing animals were
kept fasted overnight and next day each formulation were
administered orally at a dose level of 250, 500, 1000 and
2000mg/kg body weight. Rats were observed for clinical signs
of toxicity continuously for 2 hours and occasionally for further
4hours for general behavioral and finally for any mortality after
24 hours till 14 days. No mortality was observed during a time
period of 14 days.
Oral glucose tolerance test of formulation
Selection of dose: Two dose level were chosen in such a
way that one dose was approximately one-tenth of the maximum
dose used during the acute toxicity studies, second dose was the
twice that of one tenth dose (200mg/kg, 400mg/kg b.wt)
Initial Screening of all the PHF for anti-hyperglycemic
activity (oral glucose tolerance test): Formulation was
screened for anti-hyperglycemic activity to get the information
on their efficacy so that the formulation which is not effective
could be modified. Formulation was analysed for antihyperglycemic
and antihyperlipidemic activity in normal healthy
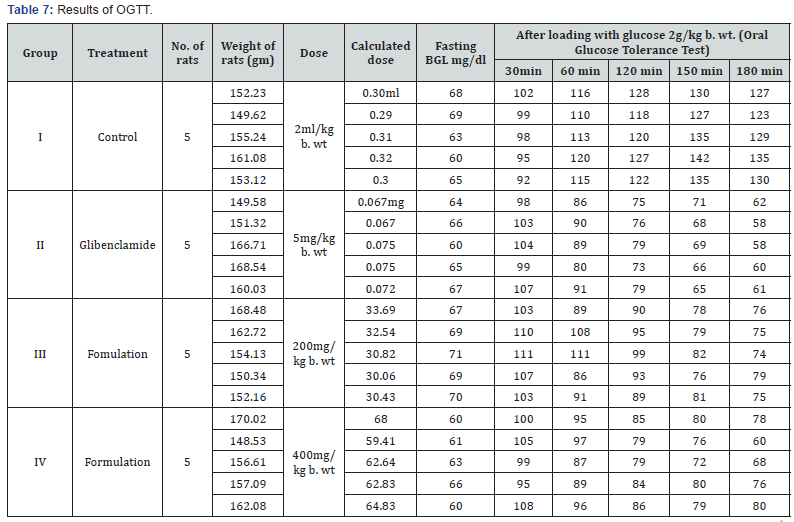
rats by conducting Oral Glucose Tolerance Test (OGTT). Initial
testing was carried out at different dose levels of formulation
(200 and 400mg/kg b. wt). Overnight fasted rats were weighed
and divided in to five groups with 5 rats in each group for each
formulation as given below. After 30 minutes, rats of all groups
were loaded orally with glucose 2g/kg b. wt. Blood glucose level
was determined by glucometer before and at 30min, 60min,
120min, 150min and 180min after loading with glucose.
Group Design for OGTT study:
Group I – Normal Control treated with vehicle i.e. (2ml/kg)
distilled water
Group II- Standard given Glibenclamide (5mg/b. wt)
Group III- treated orally with F-A 200mg/kg b.wt.
Group IV- treated orally with F-A 400mg/kg b.wt.
Antidiabetic activity
Study protocol: Induction of diabetes and experimental
study Diabetes was induced in rats by intra-peritoneal injection
of Streptozotocin (45mg/kg b. wt) which was dissolved in normal
saline. After 72h of STZ administration blood glucose level was
measured by one touch glucometer (Johnson & Johnson, India)
to confirm diabetes. Blood samples were drawn by picking the
rat tail. The diabetic rats with blood glucose levels ≥250mg/dl
were selected for the studies. After 72hr. of STZ injection animal
with BGL≥250mg/dl were divided into different groups (with 5
animals each) for anti-diabetic study of Formulations. Following
groups were prepared:
Group I –Normal control (given distilled water)
Group II-Negative control (treated with STZ 45mg/kg b. wt
i.p)
Group III-Standard (Treated with Glibenclamide 5mg/kg b.
wt after 3rd day of STZ injection)
Group IV-Treated orally with Formulation A with dose of
200mg/kg b. wt after 3rd day of STZ injection
Group V- Treated orally with Formulation A dose of 400mg/
kg b. wt after 3rd day of STZ injection
Study was conducted for 14 days. Treatment was started
from 3rd day. Standard drug and Formulations given daily for
14 days and blood glucose levels were measured with the help
of one touch glucometer (Johnson & Johnson, India) on 3rd day
(assume as 0hrs.), after 3hrs., 5th day, 10th day and 14th day of
experiment. Blood sample was taken by picking the rat tail vein
and for the measurement of other biochemical parameters blood
sample was withdrawn from retro orbital plexus of rats.
Assessment of Biochemical parameters: At the end of 14th
day of experiment, 2-4ml blood sample was withdrawn from
retro-orbital plexus of rats and centrifuged at the 5000rpm for
15-20min; serum was separated and taken out with the help
of syringe. Serum of rats was used for the analysis of other
biochemical parameters through Auto analyser.
Results and Observation
Physiochemical evaluation of plant materials
It was observed that all physicochemical evaluation
parameters contain i.e. foreign organic matter, Total ash, Acid insoluble ash, Alcohol extractive and water-soluble extractives
of plant drug was found to be within Ayurvedic pharmacopeia
limits Table 2.
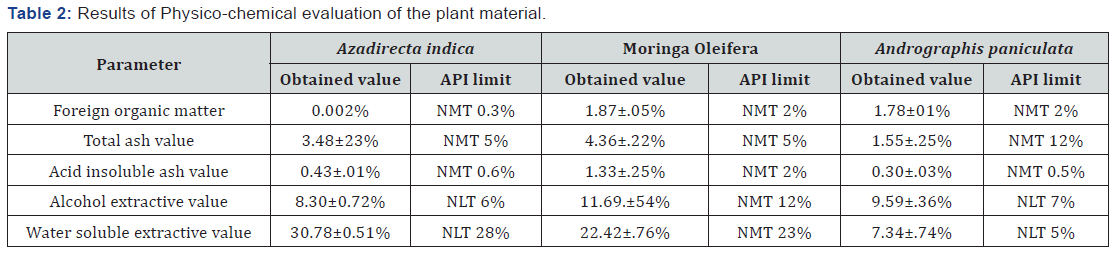
(NMT-Not more than, NLT –Not less than).
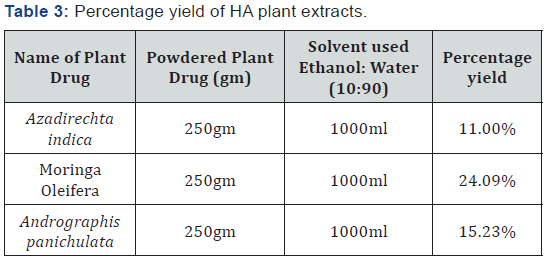
Percentage yield of all the HA plant extracts
The percentage yields of all HA plant extract are given in
Table 3.
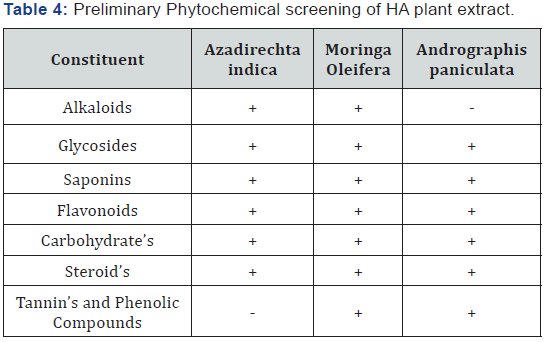
Preliminary phytochemical screening of HA plant extracts
Results of phytochemical screening are shown in Table
4. It was found that Azadirechta indica, Moringa Oleifera and
Andrographis paniculata contain all tested phytochemical
compounds.
Design and development of PHF
PHF was made in such a way so that it covers most of
targeted sites in body to decrease the blood glucose level for
their anti-diabetic action. For formulations quantity of doses
used in developing the formulation was calculated on the basis
of therapeutic doses reported in literatures.
Evaluation of polyherbal formulations: The various
combinations of dried powdered extracts of Azadirechta indica,
Moringa Oleifera, Andrographis paniculata were prepared
and evaluated on the parameters like angle of repose, loose
bulk density, tapped bulk density, carr’s index and hausner
ratio. Preformulation study of the granules showed that all the
evaluated parameters were within the acceptable limit Table 5.
Acute toxicity study of PHF formulation
STZ induced diabetic rats treated with PHF did not show any
discernible change in behaviour up to the dose level of 2000mg/
kg body weight. No sign of mortality was observed during the
observation of 14 days Table 6.
Oral glucose tolerance test (OGTT) of PHF
At 30min after the administration of 2gm/kg glucose orally,
the plasma glucose level is significantly increased and the blood
glucose level decreases gradually with the administration of
formulations. Results are given in Table 6 and results expressed
in Mean±SD in Table 7.
Findings of OGTT study: It was found that PHF with
dose of 200mg/kg body weight showed effective decrease in
blood glucose i.e. 75.75±1.92mg/dl and dose 400mg showed
72±2.73mg/dl at 180min. From the study it was predicted that
PHF possess Anti-hyperglycemic activity.
Antidiabetic activity
Experimental study: Albino wistar rats of either sex
(150-180gm body weight) were used for this study; they were
acclimatized and given proper diet. The study was approved
by the Institute Animal Ethics Committee and all the animal
experiments were carried out according to the Committee for the
Purpose of Control and Supervision of Experiments on Animals
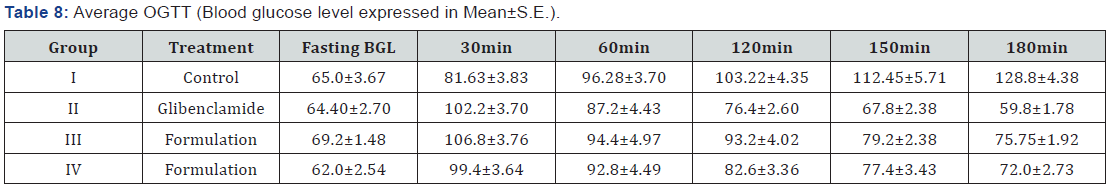
(CPCSEA) guidelines. Results showed the significantly increase in blood
glucose level in STZ treated diabetic rats. Glucose levels
measured in blood of normal and experimental rats are given
in Table 8. On repeated administration of vehicle, PHF and
glibenclamide for 14 days, a sustained and significant decrease
in the average blood glucose level of diabetic rats was observed.
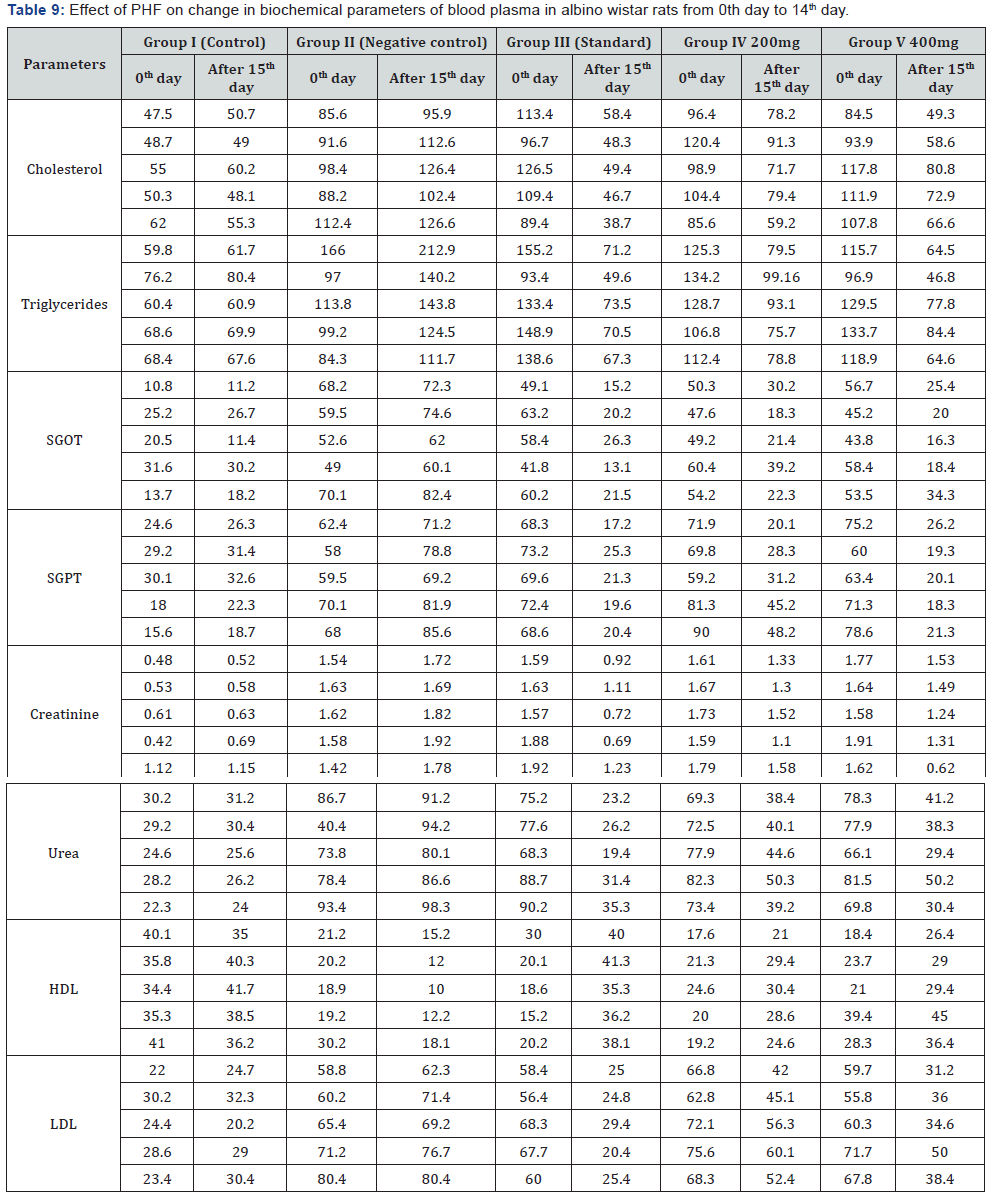
Biochemical parameters: Serum TG, Total cholesterol,
LDL cholesterol were found to be increased significantly
(P< 0.0001) in STZ induced diabetic rats (shown in Table 9) as
compared to non-diabetic control. HDL cholesterol was found
to be significantly decreased in diabetic rats. Treatment with
PHF produces a significant reduction in elevated serum TG, TC,
LDL-cholesterol level in diabetic rats. In Biochemical Parameters
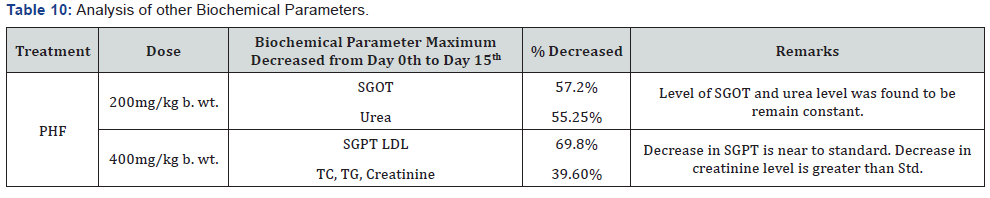
PHF (400mg and 200mg) showed maximum decrease in SGPT,
Urea and LDL Cholesterol level i.e. 69.8% near to glibenclamide,
43.36% and 39.6% Table 10.
Discussion
PHF have been developed with combinations of (3 Plants)
antidiabetic activity was investigated in albino wistar rats with glibenclamide as standard, STZ was used to induce diabetes
in rats. Formulation showed significant decrease in Blood
glucose level with improvement in slight loss of body weight,
Albino wistar rats were divided into V groups with n=5 and the
diabetic rats received the formulation, vehicle and standard
drug. Although formulation showed good antidiabetic activity. It
showed 65.8% decrease in average blood glucose level which was
very closer to standard drug glibenclamide. i.e. 66.2%. Reason for
this superior activity of Formulation may be its potential active
constituents which could possess better antidiabetic activity
and the second main reason may its synergism (herb-herb
interactions) which may be more compatible when formulated
together and thus produce more effective results. As mentioned
in results all the formulations give dose dependent antidiabetic
effect in this combination of medicinal plants. It was proved to
be fruitful and comparable to standard against glibenclamide.
PHF showed good antidiabetic activity with dose of 400mg (i.e.
62.4 %) decrease in blood glucose level. On the basis of best
synergistic effect, the lipid content except HDL was found to be
increased in STZ diabetic rats. HDL Cholesterol was found to be
more increased in combination as compared to individual. All
combinations improve the conditions of hypercholesterolemia.
PHF showed a greater increase in HDL % level to 57.12 % than
those of standard. It has been observed through literatures that
plants constituents like glycosides, alkaloids, flavonoids all these
constituents have proved to be strong antidiabetic agent through
different mechanism.
Conclusion and Direction for Future Use
Since Ancient times medicinal plants as single drug and in
combination with other herbal drugs are using in the treatment
of various chronic and non-chronic disorders. Ayurveda is one
of the most traditional systems of medicine which describes
the methodology to use the medicinal plants as healing power
in treating the disease. Polyherbalism is also the best concept
of Ayurveda, which consists of magical power of healing the
disease. Ayurveda is one of the reliable and trustworthy medicine
systems. In developing countries mostly 75-95% of populations
rely on herbal drugs. Deep research and investigation still
needed on this magical system of medicines. Research Studies
pertaining to safety, toxicological studies, Standardization,
clinical trial studies are still required to grow Ayurveda and
increasing its wide acceptability. Numbers of commercialized
standardized herbal drugs are quiet less in market since we are
lacking in developing the regulatory standards implemented
protocols. Diabetes mellitus has appearing as dreadful disorder
for society. It directly impacts our metabolic system by making
it sluggish in catabolic activities. It is mainly characterized
by hyperglycaemia resulted from decrease insulin secretion.
This dreadful disease can lead to many more complications
like blindness, kidney failure and organ dysfunction. Several
synthetic drugs are available in market but with long use of
these drugs could lead to serious side effect including the kidney
failure there is greater risk of using these synthetic drugs for
long term. Study of ancient Ayurvedic books like Charak Samhita
and Sushastra Samhita revealed that drugs used in Ayurvedic
formulations worked synergistically on root cause of disease.
Therefore, a quality control drug will be effective in management
of diabetes. In view of above 3 plants, based on their reported
mode of action PHF was made. PHF was subjected to acute
toxicity study and found to be safe up to dose of 2000mg/kg b.wt.
After this oral glucose tolerance test (OGTT) was performed
in animal model for preliminary assessment of antidiabetic
activity. The antidiabetic activity was studied in albino wistar
rats as per standard protocol. The diabetes was induced by use
of Streptozotocin (STZ). For the study of antidiabetic activity
PHF was given in 2 doses of 200mg/kg b. wt and 400mg/kg
b.wt. for 14days.The blood samples of each rat were analysed
for various biochemical parameters. The results showed that
PHF containing extracts of (Azadirecta indica, Moringa oeifera
and Andrographis paniculata) showed significant antidiabetic
and antihyperlipidemic activity which was close to standard
drug. Along with remarkable reduction in Total Cholesterol
(TC) level and increased in High Density Lipoprotein (HDL) STZ
induced diabetes rats. The formulation has emerged as potential
combination which can challenge the synthetic drug.




Comments
Post a Comment