Development of Enpp1 Inhibitors as a Strategy to Activate Stimulator of Interferon Genes (STING) in Cancers and Other Diseases- Juniper Publishers
juniper Publishers- Journal of Cell science
Abstract
Ecto-nucleotide pyrophosphatase/phosphodiesterase-1
(ENPP1/NPP1) is a membrane-bound nucleotide metabolizing enzyme that is
implicated in a variety of physiological and pathological conditions.
Recently, ENPP1 was discovered as the dominant 2’3’-cGAMP hydrolyzing
enzyme. 2’3’-cGAMP is the endogenous STING agonist, generated from
breakdown of cytosolic DNA by cGAS. Hydrolysis resistant 2’3’-cGAMP’s
have been demonstrated to be potent activators of STING-dependent innate
immunity and these are currently undergoing clinical trials in cancer.
Here we discuss ENPP1 as a potential therapeutic target for activation
of STING-dependent innate immune response.
Keywords: Innate immunity; STING; ENPP1/NPP1; Cytokines; Immunotherapies; Interferon; T-cell priming
Introduction
Innate immunity is the first response in the human
body against pathogenic, or disease-causing stimuli. These stimuli can
vary, and include viruses, perturbed normal tissue, and dying cancer
cells. It is an important response, as it prevents continued
proliferation of these pathogens and maintains a state of homeostasis
within the body. It can also accommodate the development of a specific
induced immune response during the first, or primary infection and, can
therefore, establish inflammatory conditions. This induced response is
specific because of the many different expressions that the cell surface
gives off in the form of pattern recognition receptors, which can
identify many of the molecules of life, such as, polysaccharides,
glycoproteins, glycolipids, and nucleic acids [1].
The definition of innate immunity has altered over
time. In earlier years, it was believed that innate immune response was
premeditated. However, recent studies have shown that innate immunity is
actually a specific response that results from damage or
pathogen-associated molecular patterns (DAMP/PAMPs) [2]. In the initial
phase, the innate immune system is able to coordinate inflammatory
responses through cells of the hematopoietic compartment (neutrophils,
macrophages and monocytes) and create conditions suitable for microbial
clearance. In the second phase, other cells like dendritic cells
are able to process antigens and present them on the surface in concert
with major histocompatibility complex (MHC) to prime T-cells. This also
allows the body to more effectively fight against infections of the same
or similar type in the future. This “memory” is dependent on two
specific types of cells: natural killer (NK) cells and macrophages.
These cells provide crucial protection against reinfection in the immune
system [3]. This “memory” found in innate immune systems is present in
both vertebrate and invertebrate organisms.
Cytokines in Innate Immune Response
Cytokines are possibly the most indispensable
component of the innate immune response. Cytokines are secreted by cells
of the immune system and facilitate interaction between different types
of cells. There are many different types of cytokines, and they are
classified mainly by their biological functions. The main types of
cytokines are: interferons (INFs), interleukins (ILs), transforming
growth factors (TGFs), and tumor necrosis factors (TNFs) [4].
Interferons are the most commonly found type of cytokine in vertebrates
and mammals and are crucial to mediate antiviral defense. To date, there
have been three types of interferons discovered in vertebrates, and
specifically mammals: Types 1, 2 and 3. Type 1 IFNs typically facilitate
the antiviral response against microbial infection-causing pathogens.
Type 2 IFNs also facilitate antiviral response, but at the same time,
vitalize the process of phagocytosis and inhibit cell growth. Type
3 IFNs have been demonstrated to be strikingly similar in function
to Type 1 IFNs [5,6]. Interleukins are a type of cytokines that also
facilitate inflammatory responses in the immune system and help
to stimulate cell growth [7]. Transforming growth factors (TGFs)
regulate cell growth, help stimulate the growth of oocyte cells
(which are found in the ovum), repair wounds inflicted upon the
body, participate in immunosuppression, or reduce the activity
of the immune system when naturally required [8]. Finally,
tumor necrosis factors (TNFs) help to stimulate macrophages
as they participate in the biological process of phagocytosis [9].
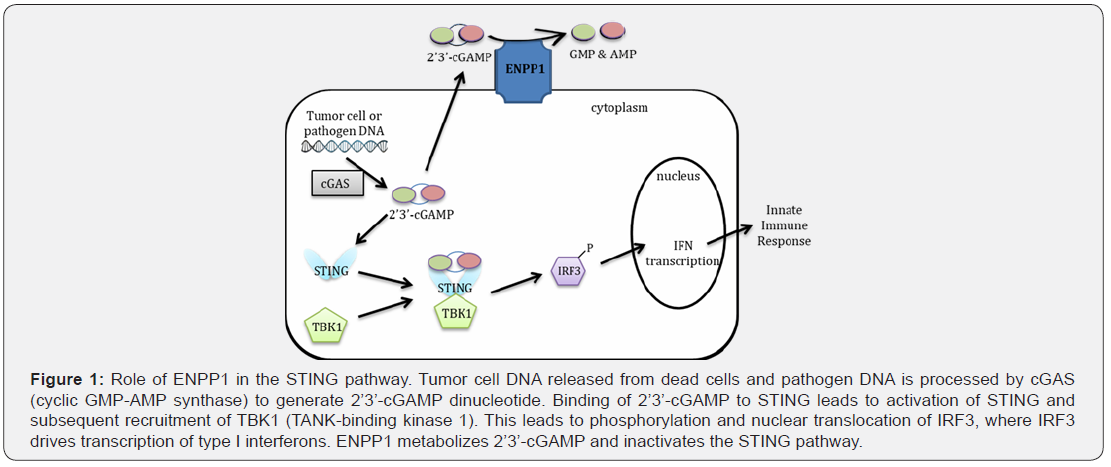
STING (Stimulator of interferon genes) as a DNA sensor
STING has been identified as a major signaling molecule
that plays a pivotal role in innate immune response by inducing
the production of interferons. STING is a cytoplasmic pattern
recognition receptor activated by nucleic acid ligands known as
cyclic dinucleotides (CDNs). These CDNs are generated by the
DNA sensor cyclic GMP-AMP synthase (cGAS) using cytosolic
DNA from extrinsic pathogens or endogenous aberrant self-DNA
[10-12]. In case of tumors, it is probable that dying tumor cells
are sources of dsDNA in the cytoplasm. In addition to CDN’s,
STING can directly sense DNA and this dual sensing has been
uncoupled with specific mutations in STING [10]. Activation of
STING induces its binding with a kinase TBK1 (TANK-binding
kinase 1) and further phosphorylation and dimerization of IRF3
(Interferon regulatory factor 3). IRF3 and another transcription
factor that is activated by STING (STAT6) translocate to nucleus
and bind to interferon promoters leading to production of type
I interferons.
It is suggested that STING pathway is the main innate
immune sensing pathway within tumor microenvironment
and the main cell types in the tumor microenvironment that
produce type I interferons are the dendritic cells [13,14]. In
addition to the activation of STING pathway in response to
tumor-derived DNA, dendritic cells prime T-cells by presenting
tumor- associated antigens. These effects then create a signaling
pathway, which allows T-cells, a main feature of the active
immune response, to neutralize tumor cells [15,16]. Some tumor
cells are able to “disguise” themselves to the innate immune
response by upregulating immune checkpoints, or by having
a lack of innate immune response within the tumor. A recent
study reported that STING is epigenetically silenced in some
cancers [17]. Additionally, oncoproteins from viruses such as
human papillomavirus can bind and block activation of STING
[18]. Thus, a cytosolic DNA sensing pathway is important for
activation of innate immune response. In recent years, there has
been considerable interest in the field of immune-oncology as
well as an increase in the number of immunotherapies available
[19,20].
ENPP1(Ectonucleotide Pyrophosphatase/Phosphodiesterase- 1) And Its Role in Innate Immunity
ENPP1 is a membrane bound enzyme that is an important
regulator of extracellular inorganic pyrophosphate in osteoblasts
and chondrocytes [21]. It is essential for prevention of soft
tissue mineralization and ENPP1 deficient mice can have abnormal
gait and progressive calcification in ectopic sites [22]. ENPP1
is responsible for hydrolysis of extracellular nucleotide triphosphates
to produce inorganic pyrophosphates (PPi) [23]. Recent
investigations have shown that ENPP1 plays a much larger role
in limiting the innate immune response of the human body. It has
been discovered that STING pathway is regulated by ENPP1[24].
ENPP1 was identified as the major hydrolase for the most potent
endogenous CDN ligand for STING: 2’3’-cGAMP [25]. Importantly,
it was demonstrated that denaturation of 2’3’-cGAMP can control
the activation of the STING pathway [26]. Phosphothioate
analogs of 2’3’-cGAMP resistant to ENPP1- mediated hydrolysis
potently activate STING [25] and mediate anti-tumor responses.
These analogs have now entered clinical trials as intra-tumoral
injections in various advance cancers (Figure 1).
In another study, it was shown that Mycobacterium tuberculosis
evades host immune response through a bacterial phosphodiesterase
(CdnP) which inactivates host 2’3’-cGAMP. Loss
of ENPP1 attenuated Mycobacterium tuberculosis infection, as
did the inhibition of CdnP, the phosphodiesterase of Mycobacterium
tuberculosis [27] More recently, inactivation of porcine
ENPP1 was shown to attenuate pseudorabies infection through
an interferon-β dependent response [28]. Many viruses generate
antagonist proteins that can inactivate cGAS-STING pathway
[29]. ENPP1 is differentially expressed in immune cells with low
levels in NK cells, DC and macrophages and high levels in neutrophils
[30]. ENPP1 is also expressed in a small subset of B-cells
and studies suggest that these cells may be involved in modulation
of T-cell activity [31]. Interestingly, ENPP1 expression was
reported to be elevated in the M2 subtype of macrophages that
are known to play a role in tumor promotion [28,32,33]. Other
studies have indicated that expression of ENPP1 is increased in
astrocytic tumors, breast cancers, and head and neck cancers
[34-36]. Thus, inhibition of ENPP1 in humans may provide opportunities
for treatment of cancers and pathogenic infections.
Challenges in Development of Inhibitors of ENPP1 for Human Use
Given the various functions for ENPP1 in regulating host
immune responses, there is interest in development of ENPP1
inhibitors for human use. These inhibitors may have promising
activity in human cancers and infectious pathologies. There are
various practical challenges in development of these inhibitors.
ENPP1 is a type II transmembrane glycoprotein that belongs to
a family of ectonucleotide pyrophosphatase/phosphodiesterase
(Enpp) family and consist of seven distinct proteins with distinct
functions [37]. Thus, any inhibitor strategy will have to consider
development challenges for specificity. In the published crystal
structure of mouse ENPP1, there are important structural
differences between ENPP2 and ENPP1. The N-terminal
somatomedin-like (SMB) domains of ENPP1 do not interact with
catalytic domains unlike those in ENPP2 [38,39]. ENPP1 appears
to lack a hydrophobic pocket in contrast to ENPP2 although
interdomain interactions are preserved [37-40]. Despite these
challenges, our group and others have described novel selective
and orally bioavailable inhibitors of ENPP1 [41-45].
Fundamental effects of ENPP1 inhibition on host immune
response are still being determined. It is not known, for
instance, if ENPP1 deficiency in mouse models impairs
anti-tumor growth. Thus, optimal duration and intensity of
ENPP1 inhibition is still being developed. This is important
since systemic administration of these inhibitors can cause
unwanted side effects due to excessive release of interferons.
Interestingly, ENPP1 knockout mice are viable, thus pointing to
possible avenues for development of such inhibitors. Prolonged
administration of ENPP1 inhibitors may lead to unwanted effects
on bony tissues and ectopic calcifications although this has been
disputed in various studies in literature [46]. This is because
bone and cartilage effects may not be entirely mediated by
ENPP1. In other studies, oral administration of pyrophosphate
can attenuate the connective tissue calcifications mediated by
ENPP1 mutations in mouse models [47].
Conclusion
As hyper-activation of STING pathway may lead to production
of abnormally high levels of proinflammatory cytokines, it is
necessary to develop therapeutics that target STING pathway
indirectly. Inhibition of ENPP1 activity is one approach that may
result in optimal activation of STING pathway, enough to have
anti-tumor effects, and minimize unintended consequences.
Given the role of ENPP1 in immune modulation and tumor
promotion, there is an increased interest to develop novel
therapies based on inhibition of the ENPP1 activity and this will
emerge as an interesting area in the coming years.
Acknowledgments
We thank Dr. Hariprasad Vankayalapati for advice in
developing this review.



Comments
Post a Comment